From 5-Azapyrimidine Chemistry to Thiadiazoles
This article is part of the series Women in Czech Chemistry
DOI:
https://doi.org/10.54779/chl20220152Keywords:
5-azacytidine, decitabine, acyclic nucleoside phosphonates, antivirals, thiadiazoles, cathepsin K, glycogen synthase kinase 3β, cystein-dependent enzymesAbstract
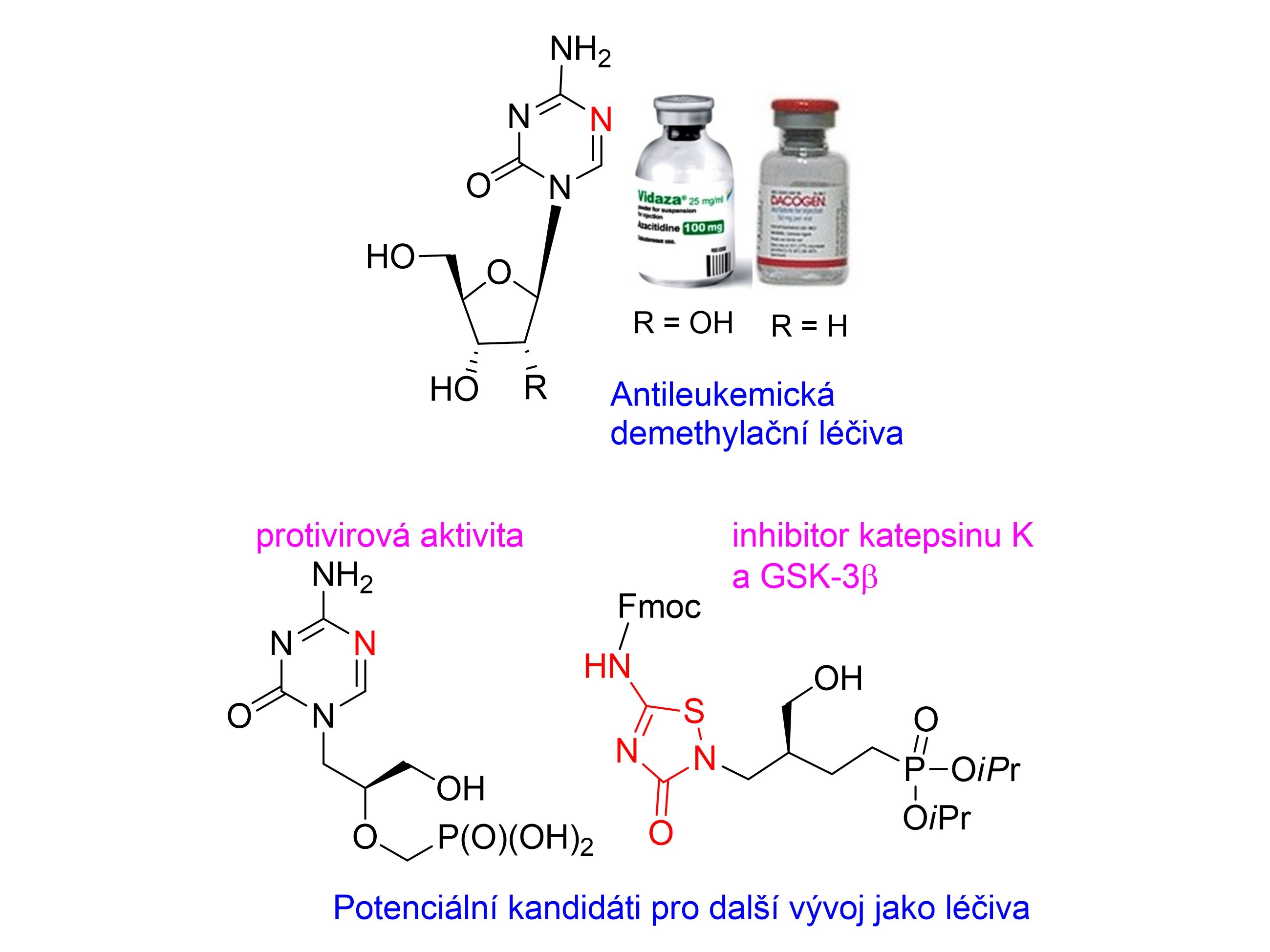
The review is devoted to 5-aza- and 6-azapyrimidine nucleosides with emphasis on antileukemic agents 5‑azacytidine and 2′-deoxy-5-azacytidine (decitabine), mechanism of their action as inhibitors of DNA methylations in tumour suppressor genes and current trends in the search for their stabilized analogues. A significant contribution to 5-azacytosine chemistry is synthesis of 5-azacytosine acyclic nucleosides phosphonates and their prodrugs, identified as potent and selective anti-DNA viral agents. Application of 5-amino-1,2,4-thiadiazol-3-(2H)-one moiety to acyclic nucleoside analogues was inspired by hypothesis that this heterocyclic base could work as a cytosine mimic and, moreover, the reactive N-S bond could be able to attack strong nucleophiles such as the cysteine thiol group of enzymes (similarly as 5‑azacytosine). A series of prepared 1,2,4-thiadiazole derivatives provided several inhibitors of Cathepsin K and glycogen synthase kinase 3β effective in the low micromolar range.